Modular Organization and Assembly of Swi/snf Family Chromatin Remodeling Complexes
Introduction
Living organisms are improbable steady-state systems. They exist in surroundings that changes from seconds to minute, mean solar day to solar day and over the years and centuries. If we inquire what enables them to continue on this unlikely class, the reply volition be "data" that they inherit in their genetic material, which allows them to have actions to prevent death. They can exercise this on diverse scales. Every organism is an agent selecting from time to time what is all-time to do in the changing circumstances. An organism can choose 'wisely' considering its 'genome' provides it with receptors tuned to respond to changes that are likely to occur. Such a diploid genome in case of man is most two meters long and must be compacted to fit into a nucleus with diameter that is about 200,000 fold smaller. And the state of affairs is quite similar in other eukaryotes. Eukaryotic cells take solved this packaging problem by folding their Deoxyribonucleic acid along with protein into a highly compacted structure called 'chromatin' [1].
Chromatin: A solution and the problem
All the chromatin proteins may be divided into two categories; histones and non-histone poly peptide. This stardom is based on unique characteristics and functions of histones. Their relative amounts and stoichiometry with respect to DNA are nearly abiding throughout the eukaryotic kingdom. Histone proteins grade a core effectually which DNA is tightly wrapped forming 'nucleosome', the structural unit of chromatin. Biochemical and genetic experiments over the past ii and a half decade accept confirmed that the organization of eukaryotic DNA in chromatin exerts a general repressive issue on replicative and transcriptional processes [ii]. Therefore subsequently solving the problem of packaging the genome, chromatin structure requite rise to another problem of accessibility of the genome by various processes requiring Dna as the substrate.
Replicative and transcriptional processes therefore require the chromatin to exist differentially unpacked and subsequently packaged, with minute temporal and spatial precision. Ane of the near intriguing phenomena related to chromatin structural variability is the presence of two morphologically different types of chromatin within a unmarried inter-phase nucleus: the dispersed euchromatin and condensed heterochromatin. The nature of replicative and transcriptional mechanics in vivo poses a tough claiming to our agreement, about how the chromatin condensation (and decondensation) is accomplished. Even though we know very little about the mechanism and nature of higher-social club chromatin folding and unfolding, the chromatin changes at a simpler (nucleosomal) level of organization have been unraveled [1]. The 10- ray crystal structure of both the histone octamer and the nucleosome cadre particles have been obtained at high resolution. During the past several years these structures have served as the main ground for interpreting nucleosome function in chromatin fibers. The nucleosome [3-five] is made up of an octamer of four core-histones H2A, H2B, H3 and H4 effectually which about 147 bp of Deoxyribonucleic acid wraps ane.65 left handed superhelical turns, assuming a molecular mass of ~206 kDa.
Different DNA binding proteins are affected differentially by the nucleosomal DNA. A common finding too many Dna binding proteins is that if its cognate DNA binding site is located close to nucleosome border then it is more attainable than the same Deoxyribonucleic acid site located shut to the nucleosomal dyad [6]. I extreme is nuclear gene NF1 which has 100-300 fold reduced affinity for its nucleosomal site compared to free DNA, contained of translational and rotational positioning. It should be noted notwithstanding, that a rotationally unfavorable positioned DNA element where the cistron binding surface, forming the base specific protein contacts, is facing towards the histone octamer, in nigh cases, commonly has drastically lower accessibility, than if this surface is positioned away from the histone surface [7].
Nucleosomes are not structurally inert but instead undergo several conformational transitions that are dynamic and probable to exist important in vivo. At molecular level nucleosomal Deoxyribonucleic acid exist in dynamic equilibrium between wound and unwound state to the histone octamer [8,9]. This dynamic behavior exposes DNA sites with a probability of 1 in 103 to 105 every bit ane moves from periphery towards the center, so the apparent Deoxyribonucleic acid binding affinities of many trans acting factors for nucleosomal Dna merely will exist reduced past 103 to 105 fold compared with the affinities of these factors for the aforementioned site on naked DNA. Thus, although the fourth dimension averaged fraction of nucleosomes with exposed binding site is small, factors having sufficiently high affinity for naked DNA and/or present in locally high concentration will still be able to demark to their cognate element in chromatin, as dictated by laws of mass activeness.
Chromatin remodeling complexes: A solution to the problem
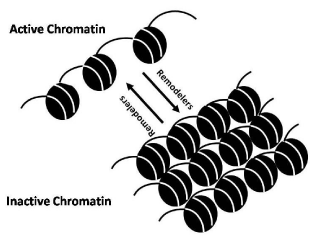
Information technology is evident that construction of nucleosome described above renders nucleosomal Dna less accessible. One molecular solution to the trouble of chromatin restructuring is provided by the activities of chromatin remodeling factors [Figure 1]. Two classes of chromatin remodeling factors have been described. First form of chromatin remodeling factors in includes protein complexes that bring about alteration in the chromatin structure by covalently modifying histones. Whereas second class remodeling complexes are of molecular motors, the ATP-dependent chromatin remodeling factors [x].
ATP-independent chromatin remodeling
ATP-independent chromatin remodeling is brought about by factors that are responsible for posttranslational, covalent modifications in various histones. Large number of elegant review articles published recently, provide in depth analysis of limerick and its functional implication of histone modifying complexes [11]. However, in order to stay focused on the theme of this thesis, chromatin remodeling by various modifications of chromatin, have been briefly addressed beneath.
Chromatin remodeling past chromatin modifications
The core histones that brand upwards nucleosome are subject to more than 100 different post translational modifications: acetylation [11], methylation [12] phosphorylation, ADP ribosylation and ubiquitilation [eleven,13]. These occur primarily at specific positions within the non-globular amino-terminal histone tails which protrude from the core of the nucleosome (explained above). Since they were discovered in 1960's histone modifications have been predicted to affect all aspects of chromosome biology, including transcription, replication, recombination and condensation, by affecting chromosome structure and by recruiting specific chromatin-binding proteins.
In that location accept seen considerable progress in understanding of acetylation and methylation of lysine residues in the histones. On a genome wide ground, histone H3 K4 tri-methylation and H3K9 acetylation are associated with agile transcriptional start sites. Phosphorylated H2A.Ten foci marking sites of Deoxyribonucleic acid harm and methylation of Histone H3 Lys9 recruits chromodomain-containing proteins, such as heterochromatin protein one (HP1) [14]. Acetylation of lysine residue in histone octamer almost always correlates with chromatin accessibility and transcriptional activity; and that the functional importance of acetylation depends completely on the accuracy and efficiency of the reverse reaction, histone deacetylation. 1 of the style in which modifications such as acetylation bear on transcription is based on the recruitment of activators due to recognition via modification binding domain for example acetylation of histones recruits bromodomain-containing proteins [15-17]. However, contempo advances in the methylation related studies indicate that lysine methylation can take different furnishings depending on which residue is modified. Methylation, in detail, was linked to the regulation of gene expression and chromatin conformation [18,19]. Near modifications are dynamic. Although histone methylation was long considered irreversible, the recent identification of numerous site-specific histone demethylases provides compelling testify that this modification is dynamically regulated [20]. A flurry of recent studies has offered glimpses into the specific biological roles of the histone modifying enzymes and their potential connections to human being diseases [20]. Of all the enzymes that modify histones, methyl transferase and kinases are the most specific.
There are two characterized mechanisms for the function of modifications. The first is disruption of contacts between nucleosomes in order to unravel chromatin and second is the recruitment of non-histone proteins. Second one is the nearly characterized till appointment. Thus depending upon the composition of modifications (histone lawmaking) on a given histone a set of proteins are encouraged to bind or are occluded from chromatin. These proteins carry with them enzymatic activities (i.east. remodeling ATPases) that further alter chromatin. The need to recruit an ordered series of enzymatic activities comes from the fact that processes regulated past modifications (transcription, replication, repair) accept several steps. Each one of these steps may crave a distinct blazon of chromatin remodeling activity and a dissimilar set of modifications to recruit them [xi].
ATP dependent chromatin remodeling
ATP dependent chromatin remodeling is brought about by the factors called remodelers. Remodelers are DNA dependent motors that apply energy derived from ATP hydrolysis to non-covalently alter this structure [21,22]. These enzymes are member of a diverse group of proteins named (SWI/SNF) subsequently the archetypal S. Cerevisiae Snf2 proteins; the Snf2 family. Multiple members of this protein family unit are nowadays in the sequenced genomes of eukaryotes, of which the chromatin remodeling enzymes form distinct sub groupings [23]. The crystal structure of catalytic domains of the ii Snf2 related proteins highlight structural similarities with the RecA domain found in the range of helicasess [24]. Snf2 proteins use the free energy of ATP hydrolysis to modify the histone Dna interaction. All the same, different bona-fide helicases, the activeness of chromatin remodeling enzymes are not by and large associated with separation of Deoxyribonucleic acid strands.
Remodelers can in vitro mediate (a) nucleosome sliding, in which the position of nucleosome on Deoxyribonucleic acid changes, (b) the creation of a remodeled state, in which Dna becomes more accessible simply histones remain bound, (c) complete dissociation of histone and DNA, or (d) histone replacement with variant histones (for a detailed give-and-take see below). At the same time, ATP dependent remodelers piece of work in conjunction with histone chaperones and histone modifying enzymes [25].
Currently, four different classes of ATP-dependent remodeling complexes can be recognized: SWI/SNF, ISWI, Mi-2, and Ino80. Each class is defined past the presence of a distinct ATPase [10].
SWI/SNF group
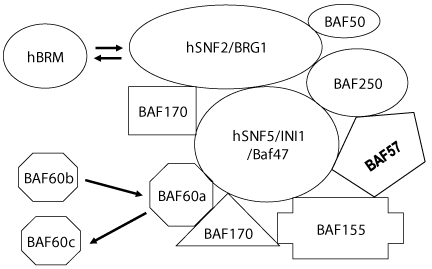
Historically, information technology was the discovery of yeast SWI/SNF complexes in the mid-1980s initiated spurt in studies of chromatin remodeling. Beginning chromatin remodeling complex was purified from yeast. It is product of five SWI and SNF gene (SWI1, SWI2/SNF2, SWI3, SNF5 and SNF6) were found to be constituents of a 2 MDa complex [26,27] named SWI/SNF complex. Later on affinity-purified complex contained, in addition to SWI1, SWI2/SNF2, SWI3, SNF5 and SNF6, 5 more then-unknown proteins with molecular weights of 78, 68, 50, 47 & 25 kDa [28].
All prototype SWI/SNF-type complexes studied so far contain a minimal structural and functional cadre composed of four evolutionarily-conserved subunits: homolog of yeast proteins SWI2/SNF2 (the ATPase, major catalytic subunit), SNF5, SWI3 and SWP73 [29,30]. The complex has an ATPase activeness that is stimulated by DNA (~30 fold) or past nucleosomes (~40 fold) [28]. Functional characterizations of the complex revealed that it could stimulate bounden of GAL4 (and GAL4 derivatives) to nucleosomal binding sites in presence of ATP. In a mutated circuitous, wherein the SWI2/SNF2-NTP bounden motif is rendered non-functional by a point mutation (K798→A), fails to stimulate activator bounden to nucleosomes. This suggests that the ATPase activity of SWI2/SNF2 is essential for the SWI/SNF office, but is not needed for structural associates of the circuitous. The circuitous was found (i) to bind Deoxyribonucleic acid in a sequence-non-specific manner with preference for iv-way junction Deoxyribonucleic acid, (2) to interact with DNA through the minor groove, and (iii) to induce positive supercoiling in relaxed plasmids in the presence of ATP [31]. The complex was however redundant when multiple transcription factors bind to nucleosomes in vitro [32]. Reportedly, the yeast SWI/SNF complex (i) disrupted a nucleosome in the presence of ATP, and (2), persistently remodeled a specific GAL4-binding site-containing nucleosome along an array of nucleosomes in presence of ATP and GAL4, and (3) evicted histones from activator-interactive nucleosomes in the presence of an activator [33]. In addition, the complex was institute to slide nucleosome forth a longer DNA fragment [34]. The available data indicate that the subunits have specific roles in determining the range of targets and biological functions of the complexes.
SWI/SNF grouping of remodelers can be further subdivided into 2 singled-out highly conserved subclasses. 1 subfamily comprises yeast SWI/SNF, Drosophila BAP (Brm associated proteins) and mammalian BAF complex; whereas the 2nd family includes yeast RSC, Drosophila PBAP, and mammalian PBAF complexes [Figure 2]. Chromatin remodeling activity although well-established across the animal phyla has likewise been reported in constitute [35].
In animals, the subunits of SWI/SNF complexes are involved in key developmental pathways at both early on and later stages of the life wheel. The mechanisms underlying this involvement include: (i) direct interactions with promoter- or enhancer-binding proteins [36], (ii) arbitration of glucocorticoid- and stress-induced apoptosis [37], (three) contribution to genomic recombination, e.chiliad., during T-cell differentiation [38], (iv) physical interactions with components of signaling pathways, e.g., interactions of BRG1 ATPase with inositol, promoted association of SWI/SNF subunits with chromatin [39,40] and (v) promoting prison cell-bike arrest, either by down regulation of E2F target genes, like cyclin E, or up-regulation of cyclin-dependent kinase inhibitors [41].
ISWI grouping
The kickoff member of this growing group of chromatin remodeling complexes, dNURF, and dCHRAC were identified in Drosophila embryo extract using in vitro assays for activities allowing transcription gene access to sites in nucleosomal arrays [42,43]. Later multiple additional remodelers belonging to this group were identified in yeast [44], humans [45,46], mouse [47] and Xenopus [48]. The ATPase subunit of this group of remodeling complex was named Imitation switch (ISWI) because of its similarity to SWI2 ATPase in the SNF2 subfamily DEAD/H helicases. Characteristic of ISWI type ATPase is the presence of a SWI3, ADA2, Due north-CoR and TFIIIB (SANT) domain and absenteeism of a bromodomain [49]. These remodeling complexes take an ATPase subunit that belongs to the SWI2/SNF2 subfamily of Dead/H helicases [50]. SANT-like domains in the catalytic subunit accept been proposed to collaborate with histone tails [51,52]. The complexes in this group are relatively smaller (300-800kDA) and comprise fewer subunits ranging from 2-4 every bit compared with larger complexes in the SNF2, CHD, and INO80 subfamilies which comprise upto 15 subunits and are often ~2MDa.
CHD/Mi2 Group
In addition to having a Swi2/Snf2-like helicase/ATPase domain, members of the CHD subfamily too contain a chromo (chromatin arrangement modifier) domain and a DNA-binding domain [53]. Chromo domains are establish in a number of proteins, including many that have the ability to interact with heterochromatin, such every bit Droshophila Polycomb, Drosophila HP1, S. pombe Clr4 histone methyltransferase (HMTase), South. pombe Swi6, and mammalian SUV39H1 HMTase. The machinery by which chromo domains are brought to heterochromatin is unclear, simply it may involve binding to methylated histones. Recently, chromo domains were plant to act every bit recognition motifs for methylated lysine-9 of histone H3 [fourteen]. Chromo domains have also been shown to interact with RNA as well as to self-acquaintance with one another [54]. Many complexes that contain a CHD family member and that display both histone deacetylase and ATP-dependent nucleosome disruption activities were purified from both humans and Xenopus laevis. In humans, this circuitous was individually identified by three different laboratories, and named NURD (nucleosome remodeling and histone deacetylation), NuRD, and NRD (nucleosome remodelling and deacetylating) [55-57]. CHD4/Mi-2h and CHD3/Mi-2a are highly related proteins that are autoantigens in the human illness dermatomyositis. These proteins are ATPases and presumably lead to the ATP-dependent chromatin-remodeling activeness of NURD complexes, and in fact recombinant human Mi-2 was found to have ATPase action comparable to intact NuRD complex [58].
A Mi-two homolog also exists in Drosophila, named dMi- ii that exists in a large complex, like to its homo and Xenopus counterparts, merely this complex is much less characterized. It does seem to contain histone deacetylase activity. Some striking differences between recombinant dMi-two and ISWI were found. The ATPase activity of dMi-2's ATPase is just stimulated by nucleosomes. Furthermore, dMi-2 was able to demark nucleosome cores (which presumably brandish no costless DNA), and dMi-2 motion histone octamers in opposite directions in a sliding assay, suggesting that in dissimilarity to ISWI remodeling complexes aMi-2 employ unlike mechanisms of nucleosome mobilization [53]
A complex highly homologous to the NURD complexes was isolated from Xenopus egg extracts that demonstrate histone deacetylase activeness along with nucleosome-stimulated ATPase action [59,60].
INO80.com
The INO80 gene (YGL150C) was identified in a genetic screen for mutants affecting inositol biosynthesis [61]. The product of this gene is highly related to the Dna-dependent ATPases in the SNF2/SWI2 superfamily of chromatin remodeling complexes. The Wu group purified and characterized the INO80 complex.
The purified INO80 complex contains 15 main subunits with roughly equivalent stoichiometry except for Rvb1 and Rvb2, which bear witness 6:i stoichiometry with other polypeptides [62]. INO80 complex is highly conserved in human INO80 complex contains orthologs of Ino80, Rvb1, Rvb2, Arp4, Arp5, Arp8, Ies2 and Ies6, as well every bit 5 unique subunits [63].
The in vitro biochemical studies showed that the INO80 complex has DNA-dependent ATPase activity, too as iii'–5' helicase action [62]. INO80.com was also found to be able to demark to costless DNA with an apparent bounden abiding (~10 nM), which is comparable to that of SWI/SNF [64]. The INO80.com participates in multiple Dna repair pathways by its nucleosome remodeling ability and by regulating the accessibility of Deoxyribonucleic acid repair proteins effectually the DSB site. Though the INO80 complex has been previously shown to play an important role in transcription, the recent finding on the roles of INO80 complex during the DNA harm response emphasizes the notion that chromatin remodeling complexes can be involved in distinctly different cellular processes [65]. DNA. INO80-C and RSC remodelers play important role in nucleosome eviction at DSB (double stranded breaks) and facilitate Rad51 bounden [66]. Additionally INO80 and RSC both remodelers announced to contribute to end resection.
Decision and Hereafter Perspective
This review discussed the problems posed past big size of genome in eukaryotes and its solution provided by packaging of the genome in the class of chromatin. However his packaging makes genome inaccessible to dissimilar factors that require DNA every bit substrate. For genome functions similar replication, repair, recombination and transcription, it requires differential unpack aging of genomic chromatin. Eukaryotic cells have solved this problem by evolving chromatin remodeling enzymes and processes that regulate chromatin structure and differential factor expression in vivo. Many chromatin remodeling complexes from yeast, Drosophila and human take been purified and characterized. Notwithstanding there are left many systems like bird and fishes where a remodeling complex is nevertheless to be purified. On one hand it tin can assistance in bridging the gap that be between identified remodeling complexes, while on the other hand it may lead to identification of novel subunits, their functions and regulation, that will add to the dynamicity of these complexes. Finally in homo health chromatin remodeling can be used for diagnosis and prognosis of traits like current and the time grade activity of disease.
Source: https://www.peertechzpublications.com/articles/GJZ-1-103.php
0 Response to "Modular Organization and Assembly of Swi/snf Family Chromatin Remodeling Complexes"
Post a Comment